Sublevels (s, p, d, f): Division of Main Energy Levels into Sublevels and Their Shapes
- Let's assume that you are walking into a massive multi-story library.
- Each floor represents a different energy level, and within each floor, there are sections labeled by genre: fiction, science, history, and more.
- Within each section, shelves hold books, neatly arranged.
Similarly, electrons in an atom are organized into energy levels (floors), sublevels (sections), and orbitals (shelves).
The Division of Main Energy Levels into Sublevels
- Electrons in an atom are not scattered randomly.
- Instead, they occupy specific energy levels around the nucleus, denoted by the principal quantum number $n$ (e.g., n = 1, 2, 3, etc.).
- Each energy level is further divided into sublevels, which represent regions where electrons are most likely to be found.
- These sublevels are labeled as s, p, d, and f, and their energy increases in the order: $$ \text{s < p < d < f} $$
Key Features of Sublevels:
- Sublevel Types:
- s (sharp): The lowest energy sublevel.
- p (principal): Higher energy than s.
- d (diffuse): Higher energy than p.
- f (fundamental): The highest energy of the four.
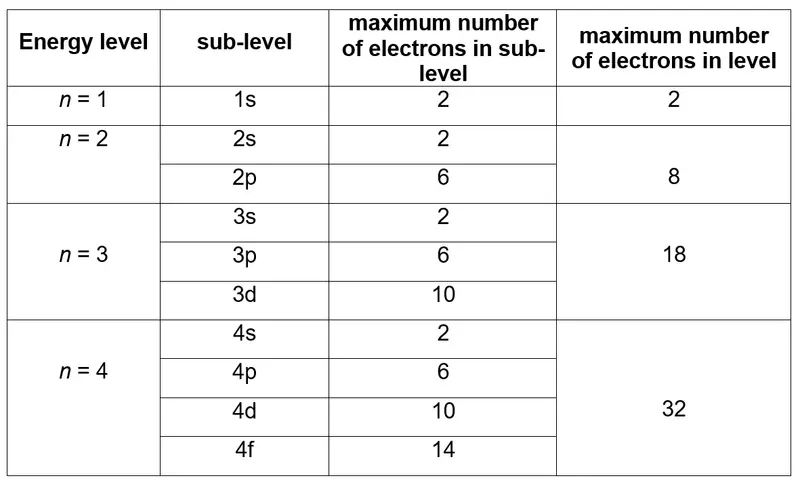
- Number of Sublevels: The number of sublevels in a given energy level equals the value of n:
- For n = 1, only the s sublevel exists.
- For n = 2, there are two sublevels: s and p.
- For n = 3, there are three sublevels: s, p, and d.
- For n = 4, there are four sublevels: s, p, d, and f.
- Maximum Electrons in a Sublevel: Each sublevel can hold a specific number of electrons:
- s: 2 electrons (1 orbital).
- p: 6 electrons (3 orbitals).
- d: 10 electrons (5 orbitals).
- f: 14 electrons (7 orbitals).
Use the formula $2n^2$ to calculate the total maximum number of electrons in an energy level, where $n$ is the principal quantum number.
Shapes and Orientations of Sublevels
- Electrons do not orbit the nucleus like planets around the sun.
- Instead, they exist in regions of space called orbitals, where the probability of finding an electron is highest.
- The shapes of these orbitals depend on the type of sublevel.
The s Sublevel: Spherical Shape
- The s orbital is the simplest type of orbital.
- It is spherical, meaning electrons are most likely to be found in a spherical region around the nucleus.
- Key Features:
- Each energy level has one s orbital.
- The size of the sphere increases with the principal quantum number n, e.g. the 1s orbital is smaller than the 2s orbital.
The p Sublevel: Dumbbell Shape with Three Orientations
- The p orbitals are more complex than s orbitals and resemble dumbbells or figure eights.
- These orbitals are oriented along the three spatial axes: x, y, and z.
- As a result, there are three p orbitals in each p sublevel, labeled pₓ, pᵧ, and p𝓏.
- Key Features:
- Each p orbital has two lobes, with a region of zero probability (called a node) at the nucleus.
- The three p orbitals are degenerate, meaning they have the same energy in an isolated atom.
Higher Sublevels: d and f (Beyond SL Scope for Shapes)
- For the d and f sublevels, the orbital shapes are even more intricate, resembling cloverleaves and complex multi-lobed structures.
- These sublevels are vital for understanding the chemistry of transition metals and the lanthanide/actinide series.
While the shapes of d and f orbitals are not required for SL students, HL students should familiarize themselves with their complex geometries.
Why Do Sublevels Matter?
Understanding sublevels helps us explain:
- Electron Configurations: The arrangement of electrons in sublevels determines the chemical properties of elements.
- Chemical Bonding: The shapes and orientations of orbitals influence how atoms bond and form molecules.
- Periodic Trends: The periodic table is structured based on the filling of s, p, d, and f sublevels.
- Can you identify the shapes and orientations of the s and p orbitals?
- How do these relate to the periodic table?