Practice R1.1 Measuring enthalpy change with authentic IB Chemistry exam questions for both SL and HL students. This question bank mirrors Paper 1A, 1B, 2 structure, covering key topics like atomic structure, chemical reactions, and organic chemistry. Get instant solutions, detailed explanations, and build exam confidence with questions in the style of IB examiners.
In a simple calorimetry experiment, which assumption is usually made?
Which equation represents the standard enthalpy of atomization of bromine, ?
Ammonia is formed by the Haber process:
The diagram below shows the energy profile of this reaction.
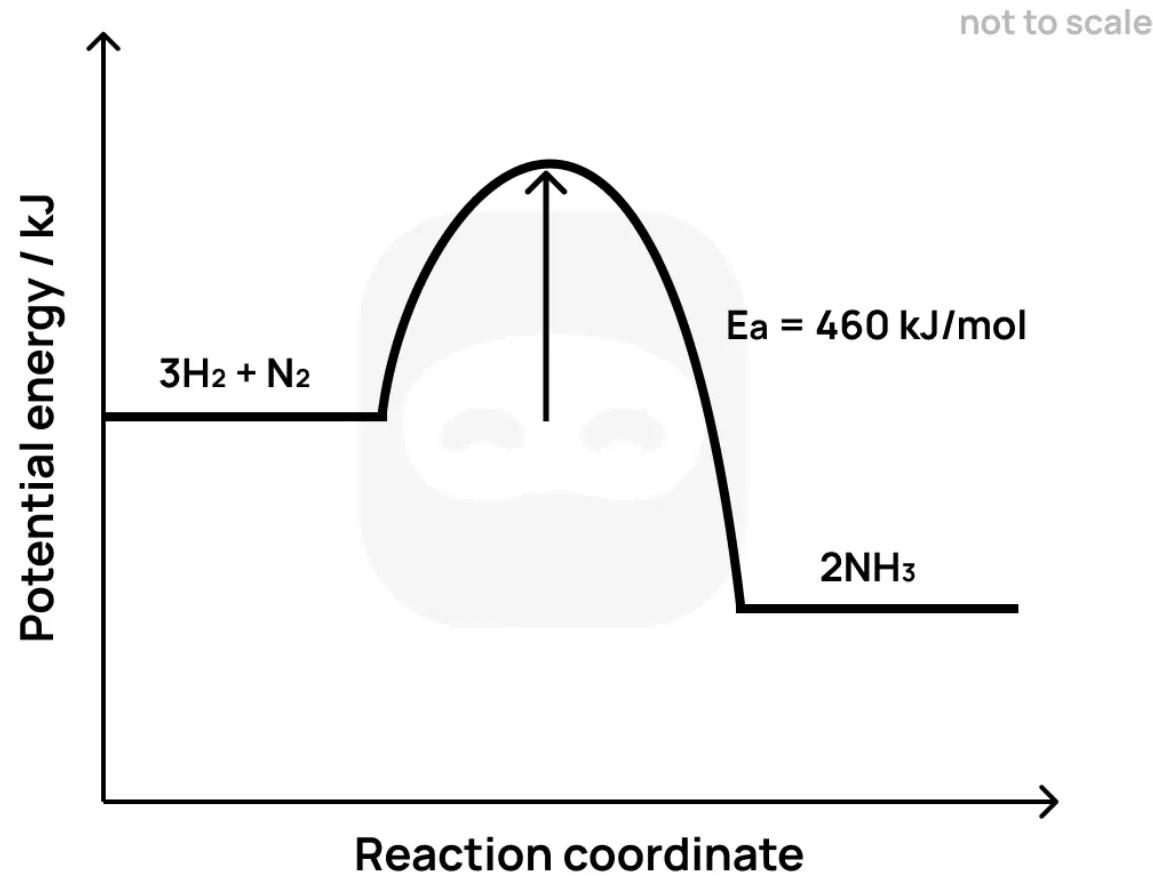
State what is meant by the term activation energy.
Identify whether the reaction is exothermic or endothermic and justify your answer using the diagram.
Suggest one reason why a high temperature is required for this reaction to proceed at a practical rate.
Explain how the use of a catalyst would affect the energy profile diagram.
The Haber process is a reversible reaction. State and explain one condition used in industry to increase the yield of ammonia.
What is the heat change, in kJ, when of aluminium is heated from to ?
Specific heat capacity of aluminium:
Which change of state is exothermic?
A student burns ethanol in a spirit burner to heat of water. The water temperature increases by . What is the heat absorbed by the water?
(Specific heat capacity of water, )
What does a negative value of indicate about a chemical reaction?
The enthalpy of combustion of ethanol is determined by heating a known mass of tap water in a glass beaker with a flame of burning ethanol.
Which will lead to the greatest error in the final result?
Which expression gives the mass, in g, of ethanol required to produce of heat upon complete combustion?
()
Which equation represents the bond enthalpy for H–Br in hydrogen bromide?