Competitive Inhibition Reduces Rate of Enzyme Activity
- Competitive inhibition is like when you're trying to charge your phone but someone keeps beating you to plugging in a cable, except it isn't connected to power at all.
- While their useless cable occupies the port, your real cable can't get in.
- The inhibitor (fake cable) fits the enzyme's active site (charging port) but can't be processed, blocking real substrate molecules from binding.
- And since only one molecule fits at a time, more inhibitors mean fewer productive reactions.
- The enzyme isn't broken, it's just wasting time on molecules that look right but don't work.
Competitive inhibition
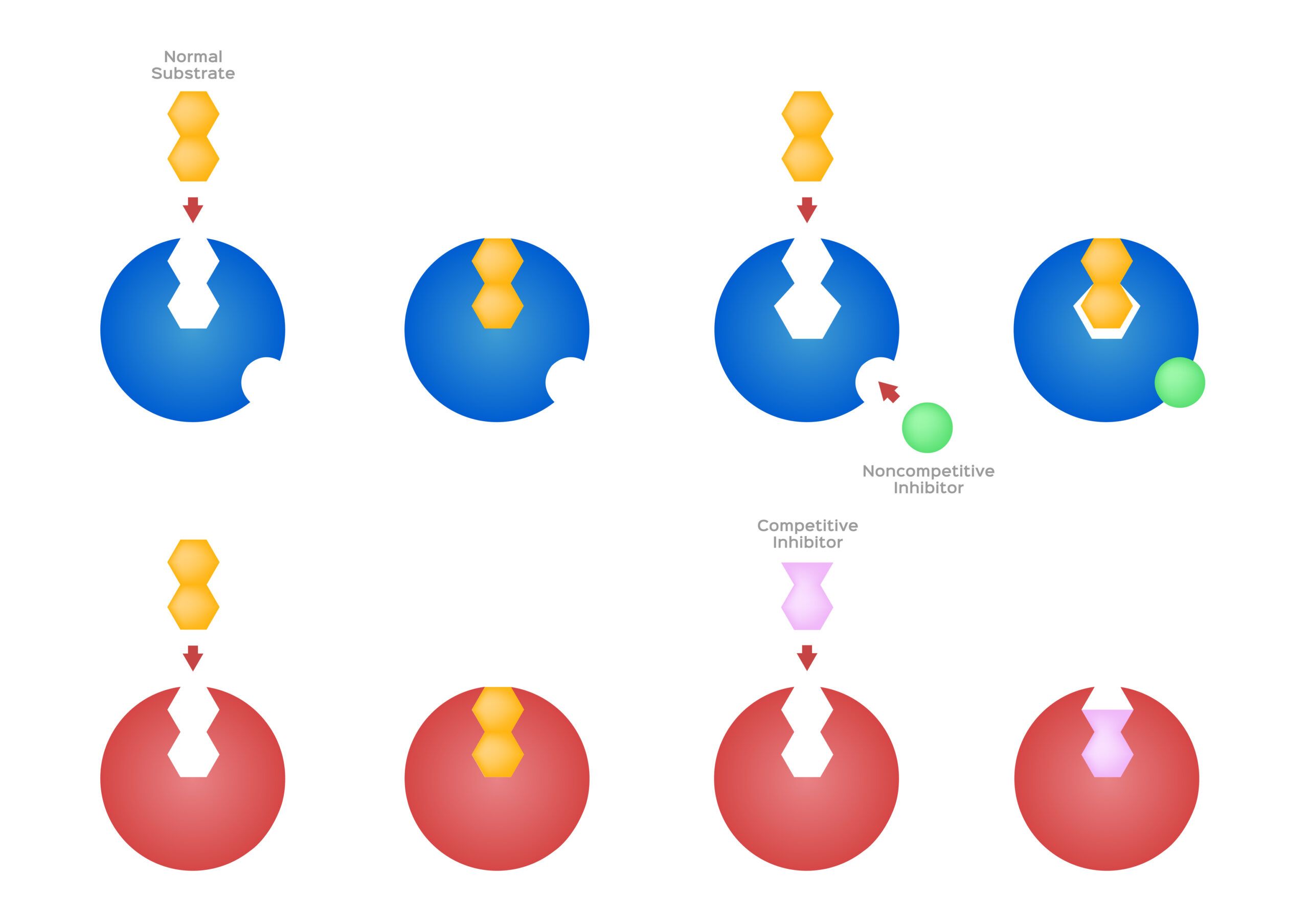
Competitive inhibition is a regulatory mechanism in enzyme-catalyzed reactions where an inhibitor molecule competes with the substrate for binding to the enzyme’s active site.
Competitive inhibitors
- Competitive inhibitors are molecules that resemble the substrate of an enzyme.
- They bind to the enzyme’s active site, blocking the actual substrate from binding.
How Competitive Inhibition Works
- In this type of inhibition, the inhibitor has a molecular shape similar to the substrate, allowing it to bind to the enzyme's active site.
- As the inhibitor occupies the active site, it prevents the substrate from binding, leading to a slower rate of reaction.
- The binding of the inhibitor is reversible, meaning that if the concentration of the substrate increases sufficiently, the substrate can outcompete the inhibitor for access to the active site.
- Therefore, increasing the concentration of the substrate can reduce the impact of competitive inhibition.
This process is reversible, meaning the inhibitor can detach, allowing the substrate to bind again.
The Role of Substrate Concentration
- The effectiveness of competitive inhibition depends on the relative concentrations of the substrate and the inhibitor:
- Low Substrate Concentration: Inhibitors have a higher chance of occupying the active site, reducing the rate of reaction.
- High Substrate Concentration: The substrate outcompetes the inhibitor for the active site, diminishing the inhibitory effect.
Increasing substrate concentration can overcome competitive inhibition, restoring the enzyme’s activity.
Statins: A Real-World Example of Competitive Inhibition
- Statins are drugs used to lower cholesterol levels in the blood.
- They achieve this by inhibiting the enzyme HMG-CoA reductase, which plays a key role in cholesterol synthesis.
- Statins mimic the structure of HMG-CoA, the enzyme’s natural substrate.
- By binding to the active site, statins prevent HMG-CoA from binding, thereby reducing cholesterol production.
- Students often confuse competitive and non-competitive inhibitors.
- Remember, competitive inhibitors bind to the active site, while non-competitive inhibitors bind elsewhere on the enzyme.
Comparing Competitive and Non-Competitive Inhibition
| Feature | Competitive Inhibition | Non-Competitive Inhibition |
|---|---|---|
| Binding site | Inhibitor binds to the active site | nhibitor binds to an allosteric site |
| Effect on enzyme activity | Reduces enzyme activity by preventing substrate binding | Reduces enzyme activity by altering enzyme structure |
| Overcoming inhibition with substrate concentration | Can be overcome by increasing substrate concentration | Cannot be overcome by increasing substrate concentration |
| Example | Statins (inhibit cholesterol synthesis) | Cyanide (inhibits cellular respiration) |
Binding Sites
- Competitive Inhibition: Inhibitors bind to the active site, directly competing with the substrate.
- Non-Competitive Inhibition: Inhibitors bind to an allosteric site, a different part of the enzyme, causing a change in the enzyme’s shape that reduces its activity.
Effect of Substrate Concentration
- Competitive Inhibition: Increasing substrate concentration can outcompete the inhibitor, restoring enzyme activity.
- Non-Competitive Inhibition: Increasing substrate concentration does not affect the inhibitor’s impact because the inhibitor does not compete for the active site.
In competitive inhibition, increasing the substrate concentration can overcome the inhibition, but in non-competitive inhibition, increasing substrate concentration does not restore enzyme activity.
- Imagine a game of musical chairs where there are only a limited number of chairs (active sites).
- The players (substrates) try to sit, but one sneaky observer (the inhibitor) grabs a chair.
- If more players (substrates) enter the game, they have a better chance of securing a chair, eventually outcompeting the inhibitor.
Non-competitive inhibitors reduce the maximum rate of reaction($V_{max}$), while competitive inhibitors do not change $V_{max}$ but increase the substrate concentration needed to reach it.
- Can you explain why increasing substrate concentration does not overcome non-competitive inhibition?
- How does this differ from competitive inhibition?