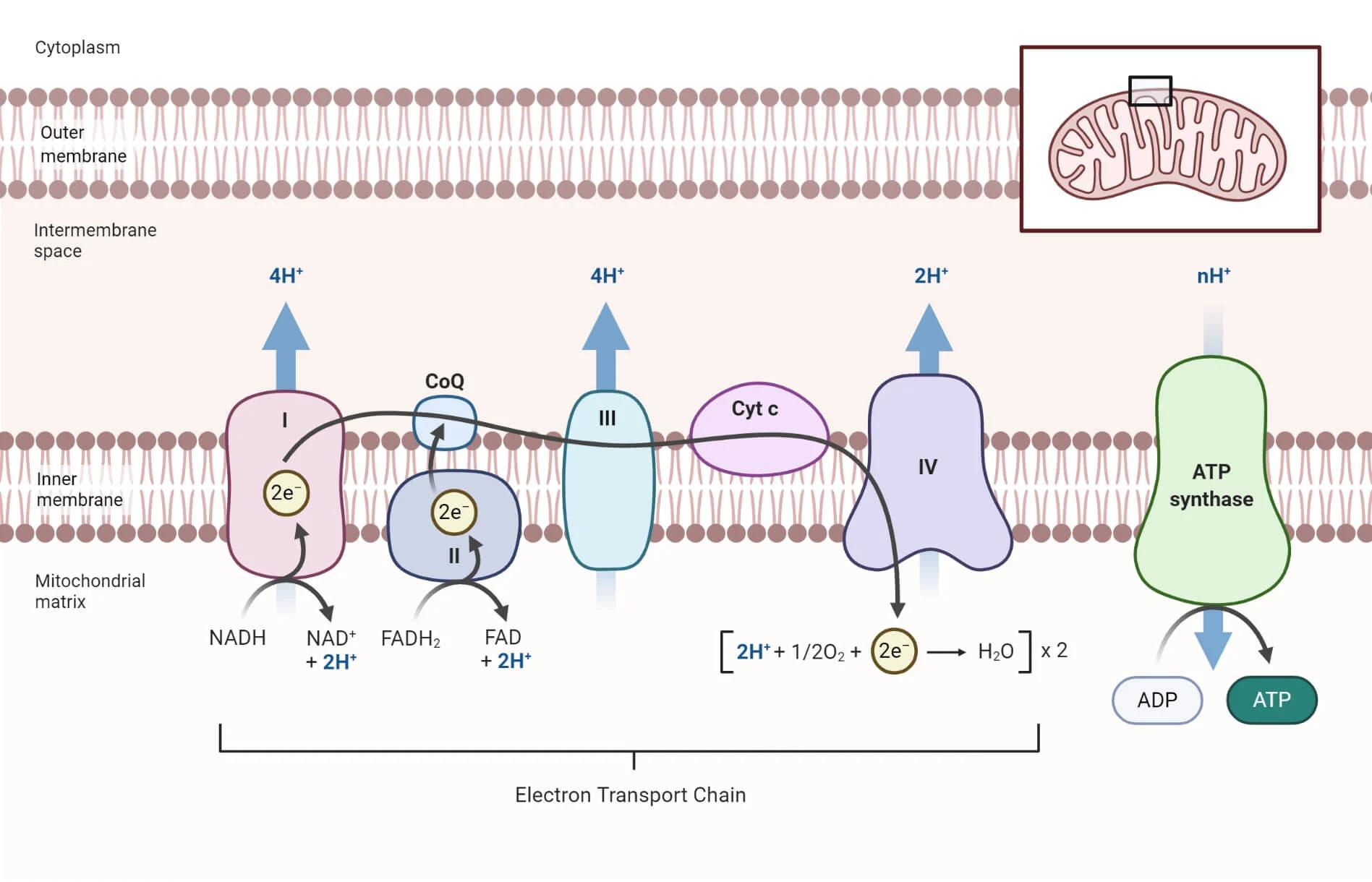
The Electron Transport Chain: A Series of Carriers
Electron transport chain (ETC)
A series of protein complexes embedded in the inner mitochondrial membrane.
After NADH and FADH₂ donate electrons to the ETC (covered in C1.2.13), those electrons flow through these carriers in sequence.
- Electrons don't release all their energy at once.
- The stepwise transfer through multiple carriers allows the cell to capture energy efficiently at each stage, rather than losing it all as heat.
Step-by-Step: How Electron Flow Generates The Proton Gradient
The electron transport chain doesn't works as a sequence of controlled transfers that efficiently extracts energy from electrons.
Step 1: Electrons Enter the Chain
- NADH donates electrons to the first carrier protein in the ETC.
- FADH₂ donates electrons to a carrier further down the chain.
- FADH₂ enters later in the chain than NADH, so it releases less energy overall.
- This is why FADH₂ results in fewer protons being pumped and less ATP produced compared to NADH.
Step 2: Electrons Move Through Carriers
- Electrons pass from one protein complex to the next.
- Each transfer involves a redox reaction, one carrier is reduced (gains electrons), then oxidized (passes electrons to the next carrier).
- As electrons pass from carrier to carrier, they move to lower energy levels.
Step 3: Energy is Released
- Energy is released at each transfer step as electrons drop to lower energy levels.
- This energy is captured by the protein complexes, it's not lost as heat.
- Electrons don't release all their energy at once.
- The stepwise transfer through multiple carriers allows the cell to capture energy efficiently at each stage, rather than losing it all as heat.
Step 4: Released Energy Drives Proton Pumping
- The protein complexes in the ETC use the captured energy to actively pump protons (H⁺) from the mitochondrial matrix into the intermembrane space.
- Protons are pumped against their concentration gradient (from low H⁺ to high H⁺).
- This is active transport, requiring energy input.
- Don't confuse where the energy comes from.
- The energy for pumping protons doesn't come from ATP, it comes from the electrons moving down the chain.
- The ETC creates the conditions for ATP synthesis; it doesn't use ATP.
Step 5: The Proton Gradient Forms
- Continuous proton pumping creates:
- High H⁺ concentration in the intermembrane space.
- Low H⁺ concentration in the matrix.
- This difference is the proton gradient (also called the electrochemical gradient).The gradient stores potential energy.
The gradient exists across the inner mitochondrial membrane, between the intermembrane space and the matrix.
- What happens to energy as electrons move through carriers in the ETC?
- How is the energy released during electron transfer used?
- In which direction are protons pumped? From matrix to intermembrane space, or vice versa?
- Where exactly is the proton gradient located in the mitochondrion?