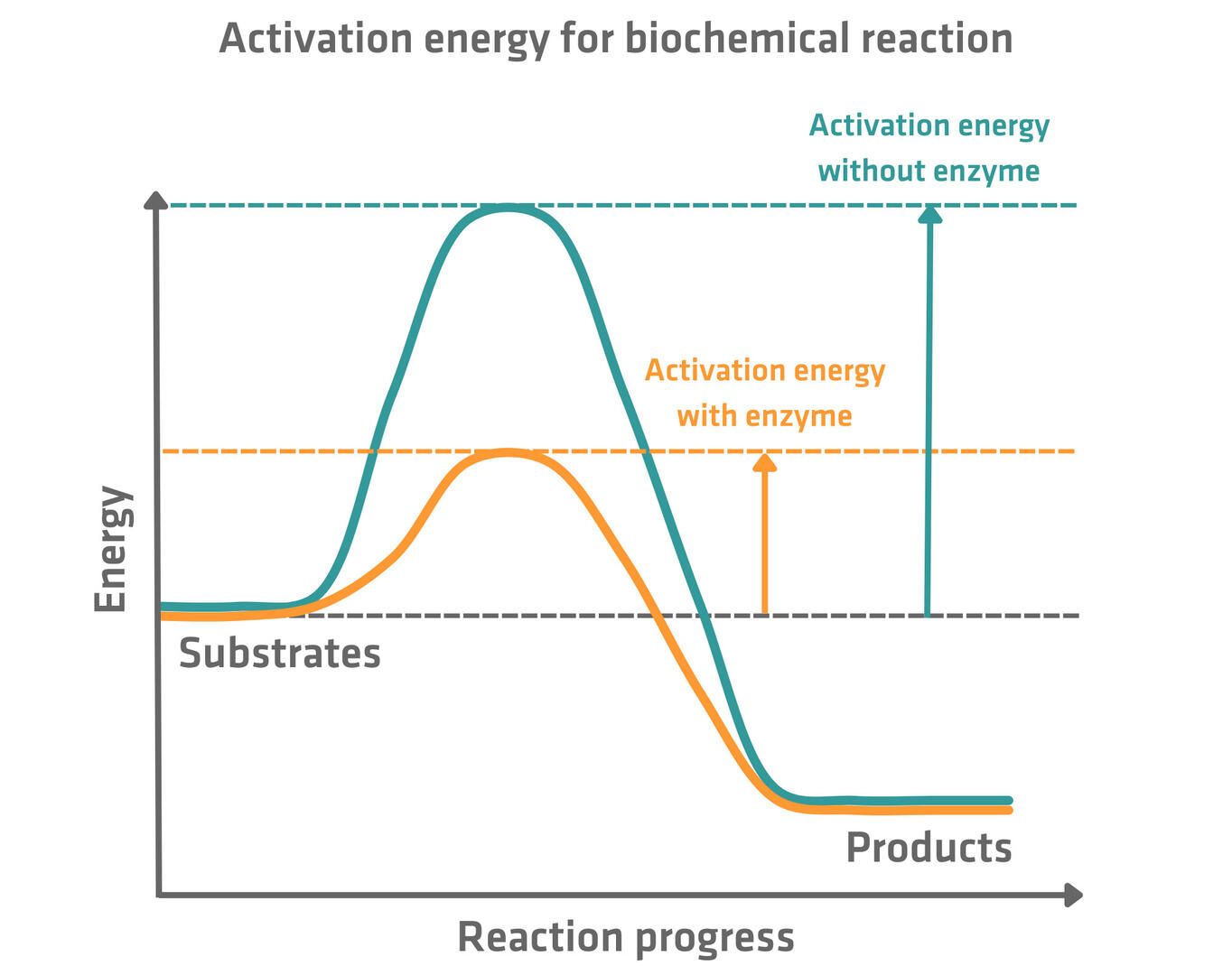
Effect of Enzymes on Activation Energy
- Consider trying to push a heavy boulder up a hill.
- The energy you exert to get it moving is like the activation energy needed to start a chemical reaction.
- Without help, this energy barrier can be too high for the reaction to occur quickly.
- This is where enzymes come in.
- The minimum amount of energy required to initiate a chemical reaction.
Think of enzymes as skilled negotiators - they lower the "cost" (energy) needed to get the reaction started.
When Is Activation Energy Low or High?
- When an enzyme is present, activation energy is low because the enzyme stabilizes the transition state, facilitating the reaction.
- This allows reactions to occur more rapidly at a lower temperature, which is essential for life processes that need to occur at body temperature.
- Without an enzyme, the activation energy is high, meaning reactions are slower unless they are heated up, which is not feasible in living organisms.
When is Activation Energy Increased?
- Certain factors, like temperature and pH, can increase the activation energy required for a reaction, particularly if they lead to enzyme denaturation (alteration of the enzyme’s shape).
- If the enzyme’s active site is disrupted, it may no longer efficiently lower the activation energy.
A high temperature could increase the energy needed for a reaction by causing the enzyme to lose its functional shape and rendering it ineffective.
- In cellular respiration, glucose is broken down into carbon dioxide and water, releasing energy stored in ATP.
- Enzymes like hexokinase and ATP synthase lower the activation energy for these reactions, making them efficient enough to sustain life.
Why Lowering Activation Energy Matters
- Faster Reactions: By reducing activation energy, enzymes allow reactions to occur at biologically relevant speeds.
- Mild Conditions: Enzymes enable reactions to proceed at normal body temperatures and pH levels, avoiding the need for extreme conditions.
- Specificity: Enzymes are highly specific, ensuring that only the intended reactions occur.
- Think of enzymes as a catalyst in a car engine.
- Without them, the fuel (substrate) would require much higher temperatures to ignite.
- Enzymes "ignite" biochemical reactions efficiently and safely.
Can you explain why the net energy change of a reaction remains the same, even when an enzyme is present?