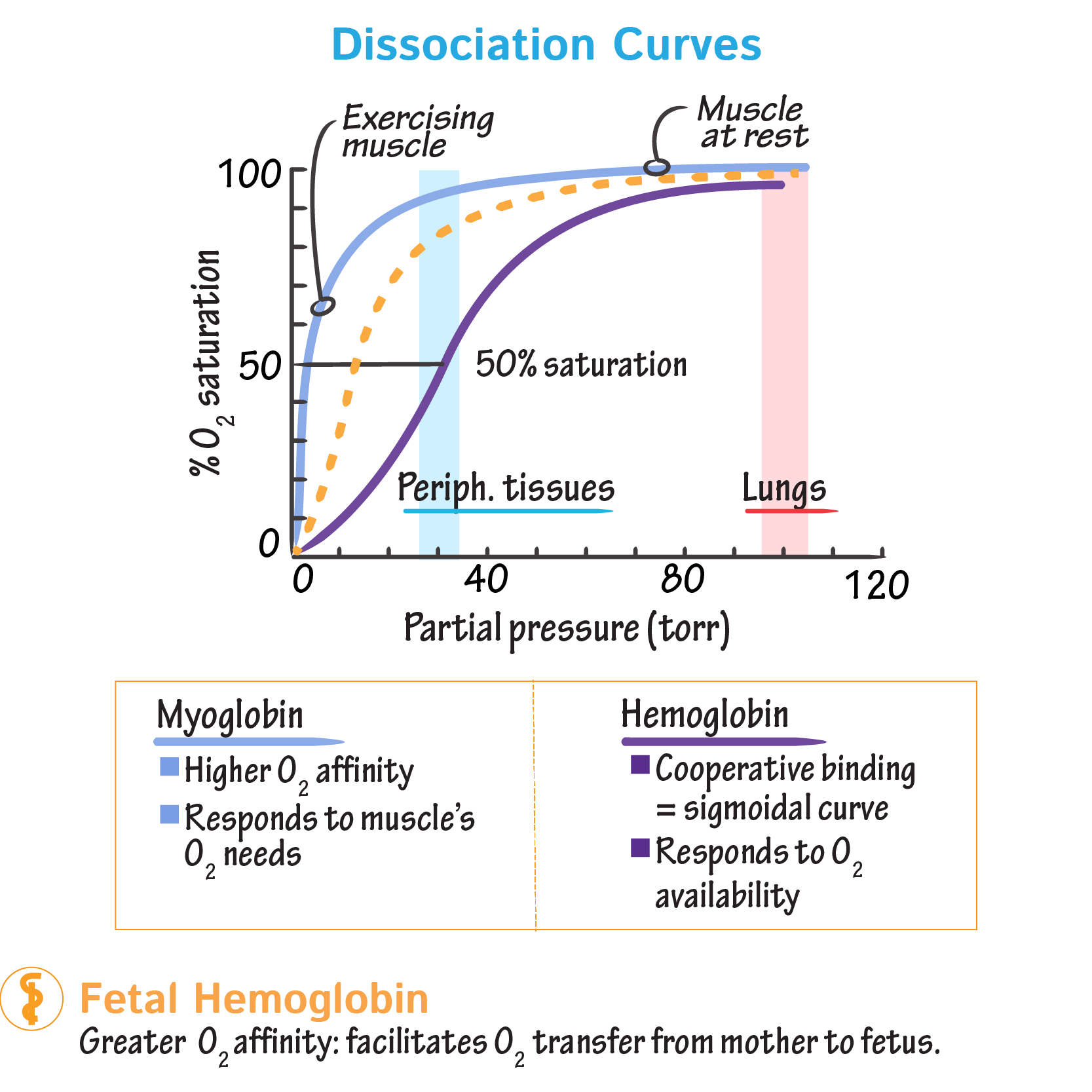
Oxygen Dissociation Curves Show Haemoglobin's Oxygen Binding and Release Dynamics
- An oxygen dissociation curve shows the relationship between the partial pressure of oxygen (pO₂) and the percentage saturation of haemoglobin with oxygen.
- It provides insights into how haemoglobin picks up oxygen in the lungs and releases it in tissues.
- X-axis: Partial pressure of oxygen (pO₂) in kilopascals (kPa).
- Y-axis: Percentage saturation of haemoglobin with oxygen.
Key Features of the Oxygen Dissociation Curve
- At high pO₂ (lungs, ~13 kPa): Haemoglobin is nearly 100% saturated, meaning most haemoglobin molecules are carrying their full complement of four oxygen molecules.
- At low pO₂ (respiring tissues, ~5 kPa): Haemoglobin releases oxygen, reducing its saturation.
- The curve is S-shaped (sigmoid) rather than linear due to cooperative binding.
This pattern allows haemoglobin to efficiently pick up oxygen in the lungs and release it where it is needed in the body.
Cooperative Binding Is The Key to the Sigmoid Shape
- Haemoglobin is a tetrameric protein, consisting of four polypeptide chains, each containing a haem group capable of binding to one oxygen molecule.
- The binding of one oxygen molecule influences the binding of subsequent molecules, making haemoglobin’s oxygen affinity dynamic rather than fixed.
How Cooperative Binding Works
- Low Affinity at Low pO₂ (Tense or "T" State):
- At low pO₂, haemoglobin has a low affinity for oxygen because the haem groups are in an inactive conformation.
- The first oxygen molecule binds with difficulty, resulting in a shallow slope at the start of the curve.
- Increasing Affinity After the First Binding (Relaxed or "R" State):
- Once the first oxygen molecule binds, haemoglobin undergoes a conformational change, making it easier for the second and third oxygen molecules to bind.
- This causes a steep increase in the curve, meaning haemoglobin can rapidly load oxygen as pO₂ rises.
- Saturation Plateau at High pO₂:
- When most binding sites are occupied, the curve levels off because fewer binding sites are available.
- The last oxygen molecule binds more slowly as there are fewer empty haem groups.
This cooperative behaviour ensures that haemoglobin is highly sensitive to small changes in pO₂, which is crucial for efficient oxygen transport and delivery.
- Imagine fastening a stiff jacket.
- The first button takes effort because the fabric resists.
- But once the first button is secured, the fabric aligns, making it easier to fasten the next buttons.
- Similarly, the binding of the first oxygen molecule makes it easier for haemoglobin to bind subsequent oxygen molecules.
The Importance of the Sigmoid Shape
- The sigmoid shape of the oxygen dissociation curve has important physiological implications:
- Maximising Oxygen Loading in the Lungs:
- In the lungs, pO₂ is high (~13 kPa), so haemoglobin is almost fully saturated with oxygen.
- This ensures the blood leaving the lungs carries the maximum possible oxygen.
- Efficient Oxygen Unloading in Tissues:
- In actively respiring tissues (e.g., muscles), pO₂ is lower (~5 kPa).
- The steep middle section of the curve ensures that even small decreases in pO₂ cause a large release of oxygen, matching metabolic demand.
- Maximising Oxygen Loading in the Lungs:
- This system allows efficient oxygen delivery to cells that need it while maintaining a reserve for less active tissues.
- The steep middle section of the curve is critical for oxygen delivery.
- It ensures that haemoglobin releases oxygen efficiently in tissues with high metabolic activity and low $pO_2$.
Comparing Haemoglobin to Myoglobin
| Feature | Haemoglonbin | Myoglobin |
|---|---|---|
| Number of oxygen-binding sites | 4 (tetrameric) | 1 (monomeric) |
| Shape of dissociation curve | Sigmoid | Hyperbolic |
| Affinity for oxygen | Variable (depends on pO₂) | High at all pO₂ |
| Function | Oxygen transport | Oxygen storage |
- Don't assume that haemoglobin always has a high affinity for oxygen.
- In reality, haemoglobin’s affinity varies depending on $pO_2$, which is why cooperative binding is so important.
Factors Influencing the Oxygen Dissociation Curve
Haemoglobin’s affinity for oxygen is not fixed, it adapts to physiological needs. Several factors shift the curve left or right, modifying oxygen loading and unloading efficiency.
1. The Bohr Effect: Rightward Shift
- Increased CO₂ or decreased pH (more H⁺ ions) reduces haemoglobin’s affinity for oxygen.
- This causes more oxygen to be released in actively respiring tissues, where CO₂ levels are high.
- This effect optimises oxygen delivery to working muscles during exercise.
2. Temperature: Rightward Shift
- Higher temperatures (e.g., during exercise) reduce oxygen affinity, promoting oxygen release in tissues.
3. Foetal Haemoglobin: Leftward Shift
- Foetal haemoglobin (HbF) has a higher oxygen affinity than adult haemoglobin.
- This allows the foetus to extract oxygen from maternal blood via the placenta.
- How does haemoglobin’s cooperative behavior challenge reductionist explanations in biology?
- Does its interplay between structure and function suggest a more holistic approach to understanding biological systems?
- Why does the oxygen dissociation curve have a sigmoid shape instead of a straight line?
- How does cooperative binding enhance haemoglobin’s function as an oxygen transporter?
- What physiological factors can shift the oxygen dissociation curve to the right or left?